

法医学杂志 ›› 2021, Vol. 37 ›› Issue (6): 796-805.DOI: 10.12116/j.issn.1004-5619.2021.310206
所属专题: 合成毒品的法医毒理学研究
张武华( ), 张明龙, 景伟伟, 谢冰, 毕海涛, 于峰, 丛斌, 马春玲, 文迪(
), 张明龙, 景伟伟, 谢冰, 毕海涛, 于峰, 丛斌, 马春玲, 文迪( )
)
收稿日期:2021-02-05
发布日期:2021-12-25
出版日期:2021-12-28
通讯作者:
文迪
作者简介:文迪,男,教授,主要从事法医毒理学研究;E-mail:wendi01125@126.com基金资助:
Wu-hua ZHANG( ), Ming-long ZHANG, Wei-wei JING, Bing XIE, Hai-tao BI, Feng YU, Bin CONG, Chun-ling MA, Di WEN(
), Ming-long ZHANG, Wei-wei JING, Bing XIE, Hai-tao BI, Feng YU, Bin CONG, Chun-ling MA, Di WEN( )
)
Received:2021-02-05
Online:2021-12-25
Published:2021-12-28
Contact:
Di WEN
摘要: 研究八肽胆囊收缩素(cholecystokinin octapeptide,CCK-8)与胆囊收缩素2受体(cholecystokinin 2 receptor,CCK2R)结合对甲基苯丙胺(methamphetamine,METH)诱导的神经元凋亡的抑制作用,并探讨β-arrestin 2在CCK-8抑制METH诱导神经元凋亡中的信号转导机制。 培养SH-SY5Y细胞和慢病毒转染的HEK293-CCK1R和HEK293-CCK2R细胞,应用干扰小RNA(small interfering RNA,siRNA)敲减β-arrestin 2的表达。采用Annexin Ⅴ-FITC/PI染色和流式细胞术检测细胞的凋亡率,Western印迹法检测凋亡相关蛋白的表达。 1 mmol/L、2 mmol/L METH可诱导SH-SY5Y细胞凋亡,核碎裂、固缩的细胞数量显著增加,凋亡相关蛋白Bax和活化型胱天蛋白酶3(cleaved caspase-3)表达升高。0.1 mmol/L和1 mmol/L CCK-8预处理均可逆转METH诱导的SH-SY5Y细胞凋亡,并抑制METH引起的核碎裂、固缩的细胞数量增多以及凋亡相关蛋白的改变。慢病毒转染构建HEK293-CCK1R和HEK293-CCK2R细胞,发现CCK-8对METH诱导HEK293-CCK1R细胞凋亡相关蛋白的变化无明显影响,但可抑制METH诱导的HEK293-CCK2R细胞凋亡相关蛋白表达水平的升高。敲减SH-SY5Y细胞的β-arrestin 2表达后可阻断CCK-8对METH诱导细胞凋亡的抑制作用。 CCK-8可与CCK2R结合,并通过激活β-arrestin 2信号抑制METH诱导的细胞凋亡。
中图分类号:
张武华, 张明龙, 景伟伟, 谢冰, 毕海涛, 于峰, 丛斌, 马春玲, 文迪. CCK-8对甲基苯丙胺诱导细胞凋亡的抑制作用[J]. 法医学杂志, 2021, 37(6): 796-805.
Wu-hua ZHANG, Ming-long ZHANG, Wei-wei JING, Bing XIE, Hai-tao BI, Feng YU, Bin CONG, Chun-ling MA, Di WEN. Inhibitory Effect of CCK-8 on Methamphetamine-Induced Apoptosis[J]. Journal of Forensic Medicine, 2021, 37(6): 796-805.
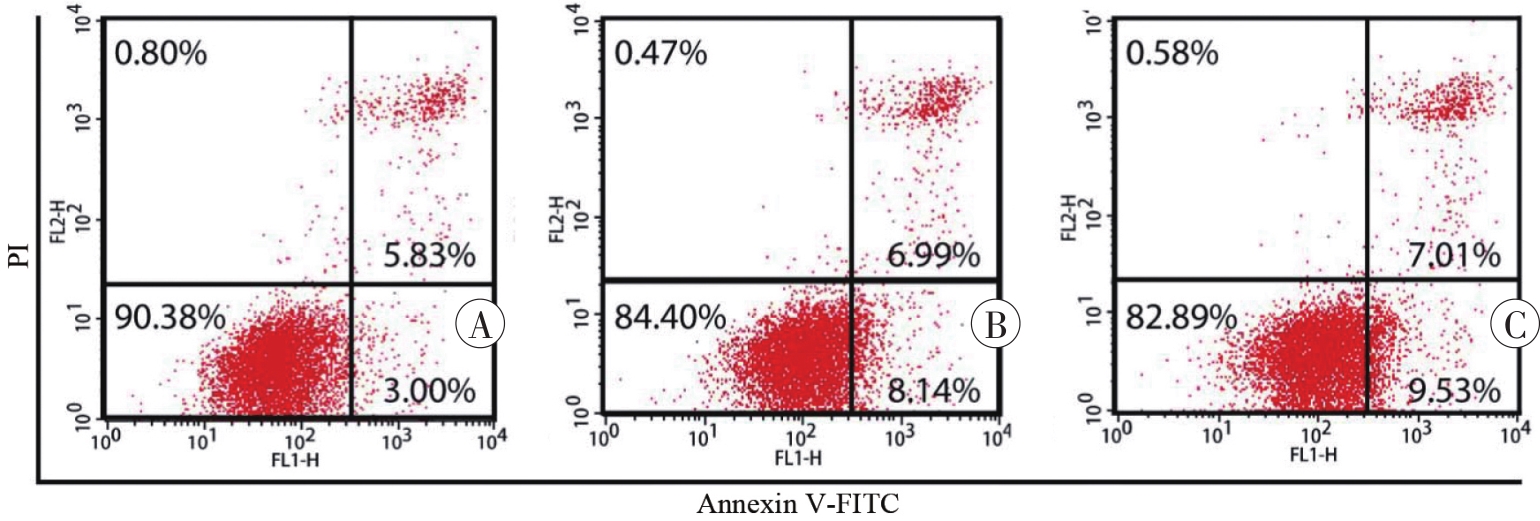
图1 Annexin Ⅴ-FITC/PI染色和流式细胞术检测SH-SY5Y细胞凋亡情况A:对照组;B:1 mmol/L METH组;C:2 mmol/L METH组。
Fig. 1 Apoptosis of SH-SY5Y cells detected by Annexin Ⅴ-FITC/PI staining and flow cytometry
| 组别 | 细胞凋亡率 |
|---|---|
| 对照 | 8.48±0.46 |
| 1 mmol/L METH | 15.05±0.921) |
| 2 mmol/L METH | 17.06±1.731) |
表1 流式细胞术检测不同浓度METH对SH-SY5Y细胞凋亡率的影响 (n=3,xˉ±s,%)
Tab. 1 The apoptosis rate of SH-SY5Y cellstreated with different concentrations ofMETH detected by flow cytometry
| 组别 | 细胞凋亡率 |
|---|---|
| 对照 | 8.48±0.46 |
| 1 mmol/L METH | 15.05±0.921) |
| 2 mmol/L METH | 17.06±1.731) |
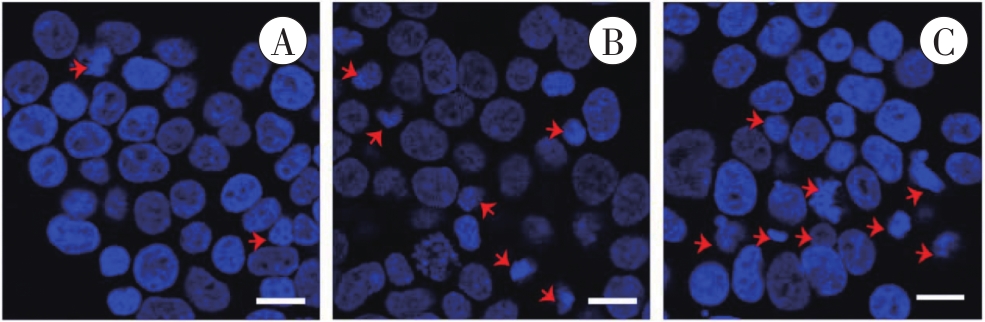
图2 Hoechst 33258染色检测凋亡细胞核的形态学改变A:对照组;B:1 mmol/L METH组;C:2 mmol/L METH组。红色箭头所示为碎裂、固缩的细胞核。Bar=25 μm。
Fig. 2 Morphological changes of apoptotic nucleidetected by Hoechst 33258 staining
| 组别 | Bax | Bcl-2 | cleaved caspase-3 | Bax/Bcl-2 |
|---|---|---|---|---|
| 0 h | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| 6 h | 1.26±0.14 | 1.08±0.16 | 1.10±0.05 | 1.26±0.31 |
| 12 h | 1.38±0.091) | 0.99±0.14 | 1.41±0.081) | 1.47±0.27 |
| 18 h | 1.56±0.051) | 0.79±0.09 | 1.46±0.061) | 2.04±0.261) |
| 24 h | 1.54±0.161) | 0.75±0.03 | 1.26±0.011) | 2.04±0.171) |
表2 METH诱导SH-SY5Y细胞凋亡相关蛋白的表达变化 (n=3,xˉ±s)
Tab. 2 Expression of apoptosis-related proteinsinduced by METH in SH-SY5Y cells
| 组别 | Bax | Bcl-2 | cleaved caspase-3 | Bax/Bcl-2 |
|---|---|---|---|---|
| 0 h | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| 6 h | 1.26±0.14 | 1.08±0.16 | 1.10±0.05 | 1.26±0.31 |
| 12 h | 1.38±0.091) | 0.99±0.14 | 1.41±0.081) | 1.47±0.27 |
| 18 h | 1.56±0.051) | 0.79±0.09 | 1.46±0.061) | 2.04±0.261) |
| 24 h | 1.54±0.161) | 0.75±0.03 | 1.26±0.011) | 2.04±0.171) |
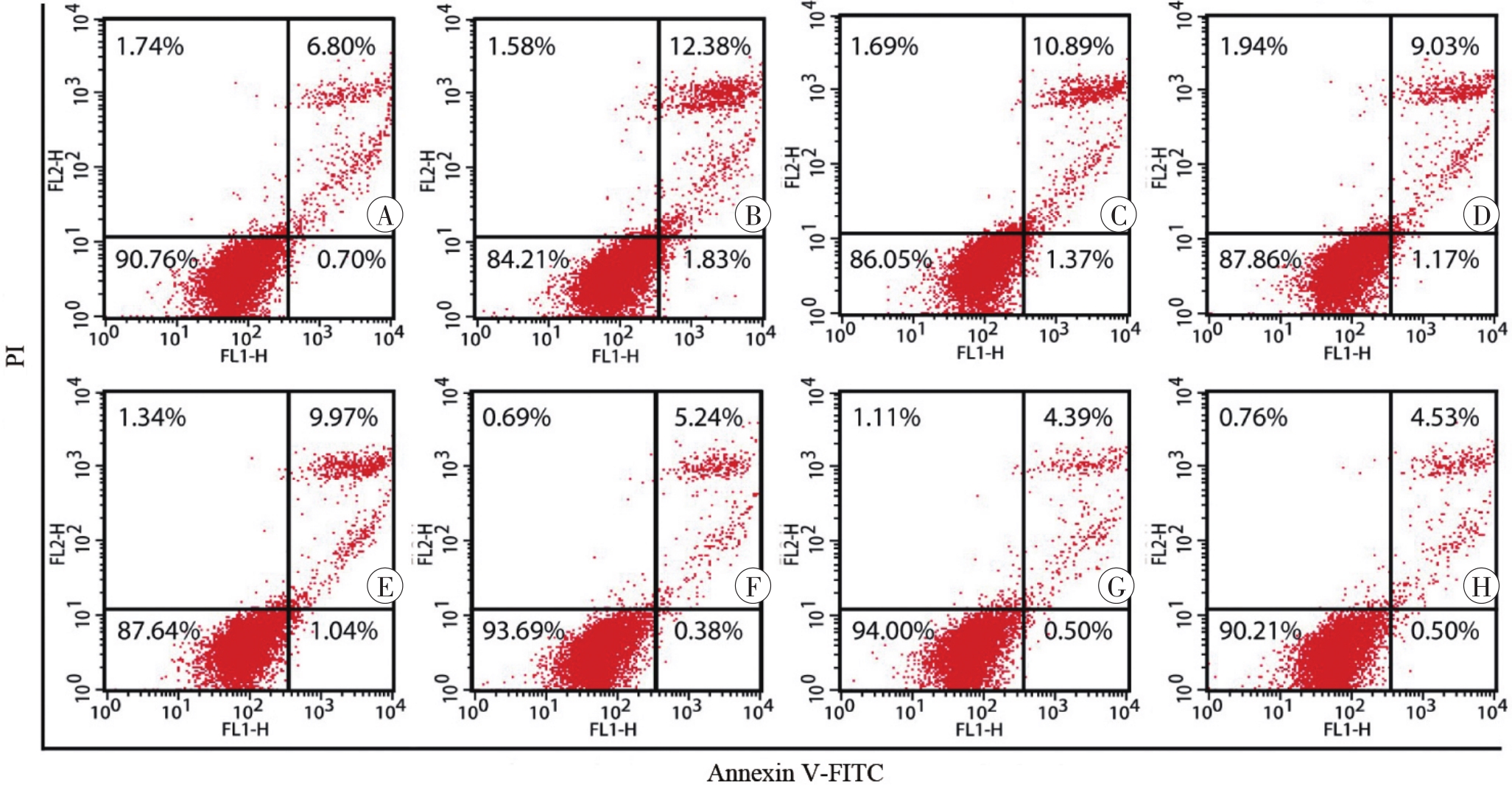
图4 Annexin Ⅴ-FITC/PI染色和流式细胞术检测SH-SY5Y细胞凋亡情况A:对照组;B:METH组;C:0.01 μmol/L CCK-8+METH组;D:0.1 μmol/L CCK-8+METH组;E:1 μmol/L CCK-8+METH组;F:0.01 μmol/L CCK-8组;G:0.1 μmol/L CCK-8组;H:1 μmol/L CCK-8组。
Fig. 4 Apoptosis of SH-SY5Y cells detected by Annexin Ⅴ-FITC/PI staining and flow cytometry
| 组别 | 细胞凋亡率 |
|---|---|
| 对照 | 8.13±0.70 |
| METH | 14.18±0.40 |
| 0.01 μmol/L CCK-8+METH | 12.20±1.24 |
| 0.1 μmol/L CCK-8+METH | 10.56±0.151) |
| 1 μmol/L CCK-8+METH | 10.77±0.291) |
| 0.01 μmol/L CCK-8 | 5.76±0.73 |
| 0.1 μmol/L CCK-8 | 5.53±0.89 |
| 1 μmol/L CCK-8 | 5.95±0.84 |
表3 流式细胞术检测不同浓度CCK-8对METH诱导SH-SY5Y细胞凋亡的影响 (n=3,xˉ±s,%)
Tab. 3 Effects of CCK-8 on the apoptosis rateinduced by METH in SH-SY5Y cellsdetected by flow cytometry
| 组别 | 细胞凋亡率 |
|---|---|
| 对照 | 8.13±0.70 |
| METH | 14.18±0.40 |
| 0.01 μmol/L CCK-8+METH | 12.20±1.24 |
| 0.1 μmol/L CCK-8+METH | 10.56±0.151) |
| 1 μmol/L CCK-8+METH | 10.77±0.291) |
| 0.01 μmol/L CCK-8 | 5.76±0.73 |
| 0.1 μmol/L CCK-8 | 5.53±0.89 |
| 1 μmol/L CCK-8 | 5.95±0.84 |
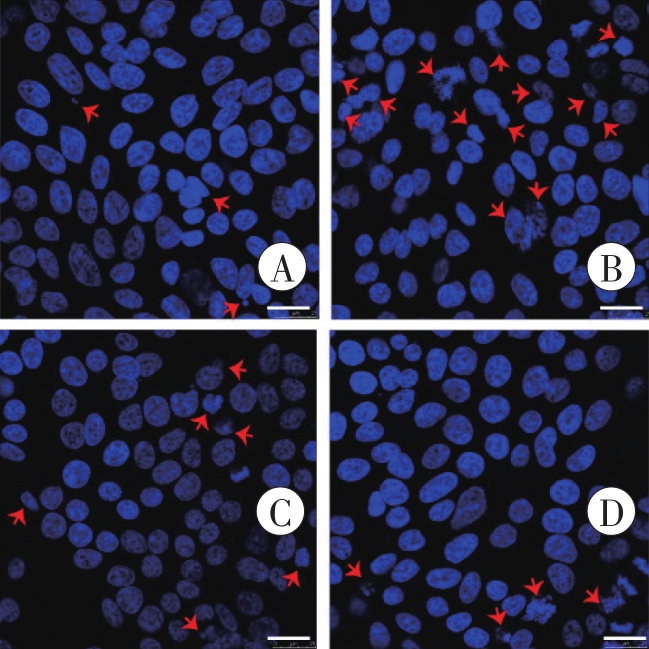
图5 Hoechst 33258染色检测凋亡细胞核的形态学变化A:对照组;B:METH组;C:1 μmol/L CCK-8+METH组;D:1 μmol/L CCK-8组。红色箭头所示为碎裂、固缩的细胞核。Bar=25 μm。
Fig. 5 Morphological changes of apoptotic nucleidetected by Hoechst 33258 staining
| 组别 | Bax | Bcl-2 | cleaved caspase-3 | Bax/Bcl-2 |
|---|---|---|---|---|
| 对照 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| METH | 1.65±0.15 | 0.90±0.04 | 1.66±0.09 | 1.83±0.10 |
| 0.01 μmol/L CCK-8+METH | 1.46±0.08 | 1.16±0.13 | 1.33±0.101) | 1.31±0.221) |
| 0.1 μmol/L CCK-8+METH | 1.33±0.111) | 1.16±0.12 | 1.23±0.021) | 1.18±0.191) |
| 1 μmol/L CCK-8+METH | 1.10±0.081) | 1.29±0.131) | 1.07±0.041) | 0.86±0.081) |
表4 CCK-8对METH诱导SH-SY5Y细胞凋亡相关蛋白的影响 (n=3,xˉ±s)
Tab. 4 Effects of CCK-8 on the expression of apoptosis-related proteins induced by METH in SH-SY5Y cells
| 组别 | Bax | Bcl-2 | cleaved caspase-3 | Bax/Bcl-2 |
|---|---|---|---|---|
| 对照 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| METH | 1.65±0.15 | 0.90±0.04 | 1.66±0.09 | 1.83±0.10 |
| 0.01 μmol/L CCK-8+METH | 1.46±0.08 | 1.16±0.13 | 1.33±0.101) | 1.31±0.221) |
| 0.1 μmol/L CCK-8+METH | 1.33±0.111) | 1.16±0.12 | 1.23±0.021) | 1.18±0.191) |
| 1 μmol/L CCK-8+METH | 1.10±0.081) | 1.29±0.131) | 1.07±0.041) | 0.86±0.081) |
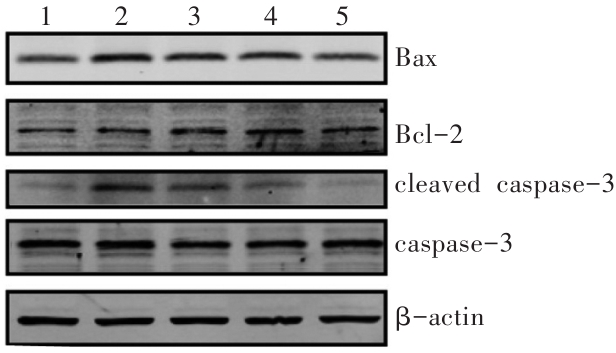
图6 Western印迹法检测CCK-8对METH诱导SH-SY5Y细胞凋亡相关蛋白的影响1:对照组;2:METH组;3:0.01 μmol/L CCK-8+METH组;4:0.1 μmol/L CCK-8+METH组;5:1 μmol/L CCK-8+METH组。
Fig. 6 Effects of CCK-8 on the expression of apoptosis-related proteins induced by METH in SH-SY5Y cellsdetected by Western blotting
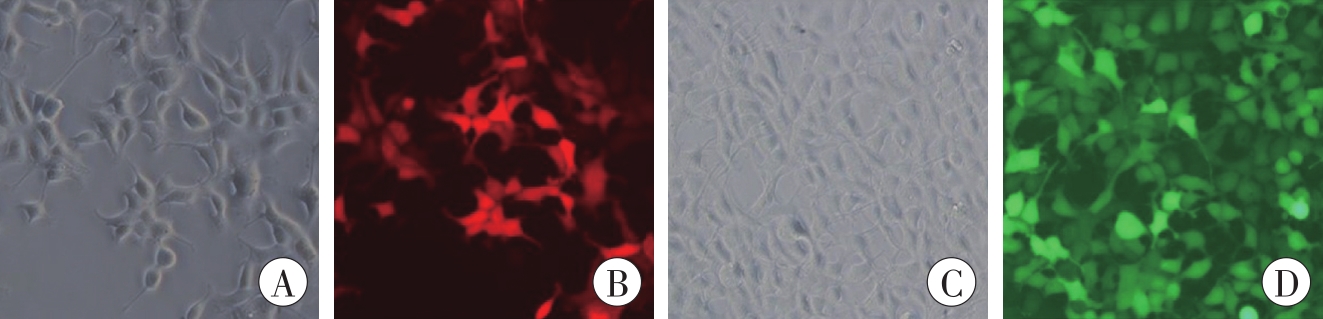
图7 明场和荧光下慢病毒空载体转染HEK293细胞的形态(×200)A、B为LV-CMV-mCherry-WPRE;C、D为LV-CMV-EGFP-WPRE。A、C为明场;B、D为荧光。
Fig. 7 Morphology of HEK293 cells transfected with lentivirus vectors under phase and fluorescence (×200)
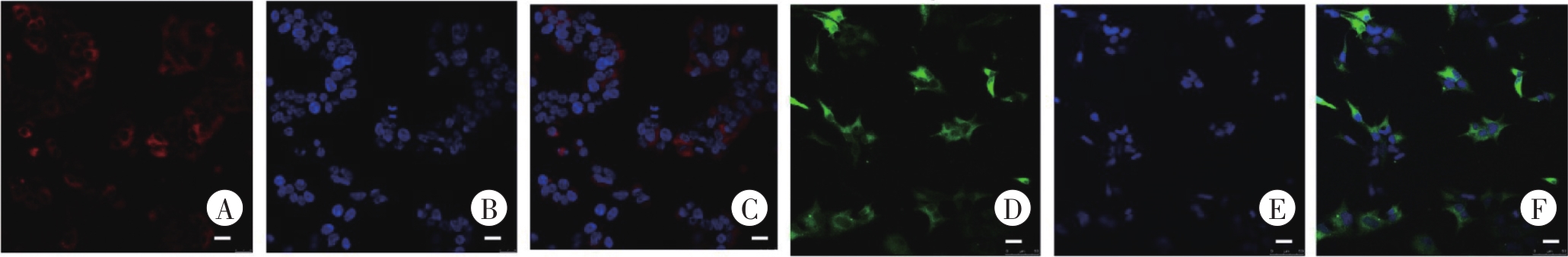
图8 慢病毒转染HEK293细胞的激光共聚焦成像A、B、C为LV-CMV-mCherry-CCK1R-Flag-WPRE-pA;D、E、F为LV-CMV-EGFP-CCK2R-HA-WPRE-pA。A、D为CCK1R和CCK2R的荧光标记;B、E为DAPI;C、F为Merge。Bar=20 μm。
Fig. 8 The confocal images of lentivirus-transfected HEK293 cells
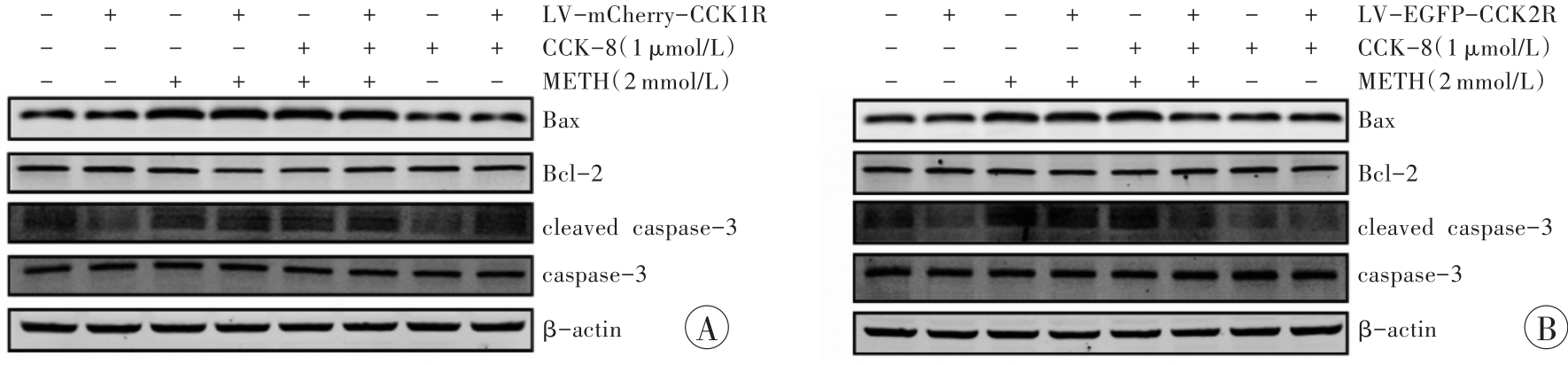
图9 Western印迹法检测CCK-8对METH诱导HEK293-CCK1R和HEK293-CCK2R细胞凋亡相关蛋白的影响A:HEK293-CCK1R;B:HEK293-CCK2R。
Fig. 9 Effects of CCK-8 on the expression of apoptosis-related proteins induced by METHin HEK293-CCK1R and HEK293-CCK2R detected by Western blotting
| 蛋白 | HEK293-CCK1R | HEK293-CCK2R | ||||||
|---|---|---|---|---|---|---|---|---|
| 对照组 | METH | CCK-8+METH | CCK-8 | 对照组 | METH | CCK-8+METH | CCK-8 | |
| Bax | 1.00±0.00 | 1.65±0.08 | 1.59±0.07 | 0.94±0.02 | 1.00±0.00 | 1.67±0.13 | 1.18±0.041) | 1.11±0.13 |
| Bcl-2 | 1.00±0.00 | 0.88±0.07 | 0.91±0.09 | 0.97±0.09 | 1.00±0.00 | 0.83±0.05 | 1.00±0.15 | 0.96±0.08 |
| cleaved caspase-3 | 1.00±0.00 | 1.85±0.14 | 1.87±0.17 | 0.99±0.17 | 1.00±0.00 | 1.76±0.06 | 1.25±0.061) | 1.02±0.10 |
| Bax/Bcl-2 | 1.00±0.00 | 1.92±0.23 | 1.80±0.24 | 0.99±0.11 | 1.00±0.00 | 2.01±0.15 | 1.23±0.151) | 1.15±0.04 |
表5 CCK-8对METH诱导HEK293-CCK1R和HEK293-CCK2R细胞凋亡相关蛋白的影响 (n=3,xˉ±s)
Tab. 5 Effects of CCK-8 on the expression of apoptosis-related proteinsinduced by METH in HEK293-CCK1R and HEK293-CCK2R
| 蛋白 | HEK293-CCK1R | HEK293-CCK2R | ||||||
|---|---|---|---|---|---|---|---|---|
| 对照组 | METH | CCK-8+METH | CCK-8 | 对照组 | METH | CCK-8+METH | CCK-8 | |
| Bax | 1.00±0.00 | 1.65±0.08 | 1.59±0.07 | 0.94±0.02 | 1.00±0.00 | 1.67±0.13 | 1.18±0.041) | 1.11±0.13 |
| Bcl-2 | 1.00±0.00 | 0.88±0.07 | 0.91±0.09 | 0.97±0.09 | 1.00±0.00 | 0.83±0.05 | 1.00±0.15 | 0.96±0.08 |
| cleaved caspase-3 | 1.00±0.00 | 1.85±0.14 | 1.87±0.17 | 0.99±0.17 | 1.00±0.00 | 1.76±0.06 | 1.25±0.061) | 1.02±0.10 |
| Bax/Bcl-2 | 1.00±0.00 | 1.92±0.23 | 1.80±0.24 | 0.99±0.11 | 1.00±0.00 | 2.01±0.15 | 1.23±0.151) | 1.15±0.04 |
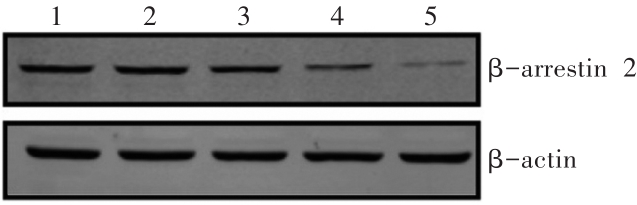
图11 Western印迹法检测不同siRNA序列转染的SH-SY5Y细胞β-arrestin 2的表达1:对照组;2:阴性对照组;3:β-arrestin 2 siRNA-1组;4:β-arrestin 2 siRNA-2组;5:β-arrestin 2 siRNA-3组。
Fig. 11 Expression of β-arrestin 2 in SH-SY5Y cellstransfected with different siRNA sequencesdetected by Western blotting
| 组别 | 相对表达量 |
|---|---|
| 对照 | 1.00±0.00 |
| 阴性对照 | 1.09±0.06 |
| β-arrestin 2 siRNA-1 | 0.96±0.06 |
| β-arrestin 2 siRNA-2 | 0.61±0.041) |
| β-arrestin 2 siRNA-3 | 0.28±0.041)2) |
表6 siRNA干扰对SH-SY5Y细胞β-arrestin 2表达的影响 (n=3,xˉ±s)
Tab. 6 Effects of siRNA on the expressionof β-arrestin 2 in SH-SY5Y cells
| 组别 | 相对表达量 |
|---|---|
| 对照 | 1.00±0.00 |
| 阴性对照 | 1.09±0.06 |
| β-arrestin 2 siRNA-1 | 0.96±0.06 |
| β-arrestin 2 siRNA-2 | 0.61±0.041) |
| β-arrestin 2 siRNA-3 | 0.28±0.041)2) |
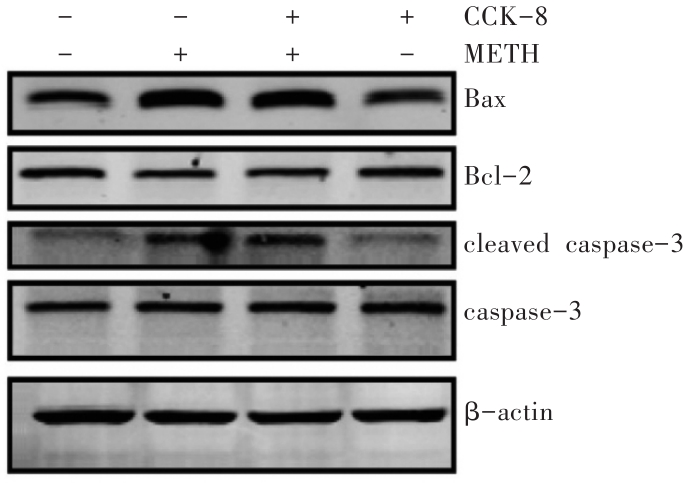
图12 Western印迹法检测CCK-8对METH诱导β-arrestin 2敲减SH-SY5Y细胞凋亡相关蛋白的影响
Fig. 12 Effects of CCK-8 on the expression ofapoptosis-related proteins induced by METHin β-arrestin 2 knockdown SH-SY5Ydetected by Western blotting
| 组别 | Bax | Bcl-2 | cleaved caspase-3 | Bax/Bcl-2 |
|---|---|---|---|---|
| 对照 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| METH | 1.81±0.041) | 0.92±0.04 | 1.80±0.071) | 1.98±0.131) |
| CCK-8+METH | 1.79±0.091) | 0.89±0.04 | 1.70±0.081) | 2.02±0.021) |
| CCK-8 | 1.07±0.02 | 1.07±0.11 | 0.91±0.05 | 1.02±0.09 |
表7 CCK-8对METH诱导β-arrestin 2敲减SH-SY5Y细胞凋亡相关蛋白的影响 (n=3,xˉ±s)
Tab. 7 Effects of CCK-8 on the expression of apoptosis-related proteinsinduced by METH in β-arrestin 2 knockdown SH-SY5Y
| 组别 | Bax | Bcl-2 | cleaved caspase-3 | Bax/Bcl-2 |
|---|---|---|---|---|
| 对照 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| METH | 1.81±0.041) | 0.92±0.04 | 1.80±0.071) | 1.98±0.131) |
| CCK-8+METH | 1.79±0.091) | 0.89±0.04 | 1.70±0.081) | 2.02±0.021) |
| CCK-8 | 1.07±0.02 | 1.07±0.11 | 0.91±0.05 | 1.02±0.09 |
| 1 | ROPEK N, AL-SERORI H, MIŠÍK M, et al. Methamphetamine (“crystal meth”) causes induction of DNA damage and chromosomal aberrations in human derived cells[J]. Food Chem Toxicol,2019,128:1-7. doi:10.1016/j.fct.2019.03.035. |
| 2 | BÜTTNER A. Review: The neuropathology of drug abuse[J]. Neuropathol Appl Neurobiol,2011,37(2):118-134. doi:10.1111/j.1365-2990.2010.01131.x. |
| 3 | WEN D, CONG B, MA C, et al. The effects of exogenous CCK-8 on the acquisition and expression of morphine-induced CPP[J]. Neurosci Lett,2012,510(1):24-28. doi:10.1016/j.neulet.2011.12.063. |
| 4 | 文迪,马春玲,丛斌,等. CCK-8与内源性阿片系统在吗啡依赖过程中的相互作用[J].中国药物依赖性杂志,2010,19(5):422-423. doi:10.13936/j.cnki.cjdd1992.2010.05.031. |
| WEN D, MA C L, CONG B, et al. Interaction of CCK-8 and endogenous opioid system in opioid dependence[J]. Zhongguo Yaowuyilaixing Zazhi,2010,19(5):422-423. | |
| 5 | WEN D, ZANG G, SUN D, et al. Effects of CCK-8 on the reinstatement of morphine-induced CPP and expression of behavioral sensitization in rats[J]. Neuroscience,2013,238:230-241. doi:10.1016/j.neuroscience.2013.02.057. |
| 6 | WEN D, AN M, GOU H, et al. Cholecystokinin-8 inhibits methamphetamine-induced neurotoxicity via an anti-oxidative stress pathway[J]. Neurotoxicology,2016,57:31-38. doi:10.1016/j.neuro.2016.08.008. |
| 7 | SUN D L, QI Y X, YANG T, et al. Early oral nutrition improves postoperative ileus through the TRPA1/CCK1-R-mediated mast cell-nerve axis[J]. Ann Transl Med,2020,8(5):179. doi:10.21037/atm.2020.01.95. |
| 8 | YANG X, WANG Y, LI Q, et al. The main molecular mechanisms underlying methamphetamine- induced neurotoxicity and implications for pharmacological treatment[J]. Front Mol Neurosci,2018,11:186. doi:10.3389/fnmol.2018.00186. |
| 9 | LI S, NI Z, CONG B, et al. CCK-8 inhibits LPS-induced IL-1β production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-κB pathway[J]. Shock,2007,27(6):678-686. doi:10.1097/shk.0b013e3180ze26dd. |
| 10 | 冯波,王蓉,盛树力. 神经退行性疾病研究中拟神经细胞模型:人神经母细胞瘤株SH-SY5Y的来源特性及应用[J].中国临床康复,2006,10(6):121-123. |
| FENG B, WANG R, SHENG S L. Mimic model of nerve cells in the study of neurodegenerative disease: Origin, characteristics and application of human neuroblastoma cell line SH-SY5Y[J]. Zhongguo Linchuang Kangfu,2006,10(6):121-123. | |
| 11 | HU X L, OLSSON T, JOHANSSON I M, et al. Dynamic changes of the anti- and pro-apoptotic proteins Bcl-w, Bcl-2, and Bax with Smac/Diablo mitochondrial release after photothrombotic ring stroke in rats[J]. Eur J Neurosci,2004,20(5):1177-1188. doi:10.1111/j.1460-9568.2004.03554.x. |
| 12 | CHOUDHARY G S, AL-HARBI S, ALMASAN A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis[J]. Methods Mol Biol,2015,1219:1-9. doi:10.1007/978-1-4939-1661-0_1. |
| 13 | WISESSMITH W, PHANSUWAN-PUJITO P, GOVI-TRAPONG P, et al. Melatonin reduces induction of Bax, caspase and cell death in methamphetamine-treated human neuroblastoma SH-SY5Y cultured cells[J]. J Pineal Res,2009,46(4):433-440. doi:10.1111/j.1600-079X.2009.00680.x. |
| 14 | TOSCANO E C B, VIEIRA É L M, PORTELA A C D C, et al. Bcl-2/Bax ratio increase does not prevent apoptosis of glia and granular neurons in patients with temporal lobe epilepsy[J]. Neuropatholo-gy,2019,39(5):348-357. doi:10.1111/neup.12592. |
| 15 | YE G, TAO L, MA C, et al. Influences of CCK-8 on expressions of apoptosis-related genes in prefrontal cortex neurons of morphine-relapse rats[J]. Neurosci Lett,2016,631:115-121. doi:10.1016/j.neulet.2016.08.028. |
| 16 | WEN D, MA C L, ZHANG Y J, et al. Cholecystokinin receptor-1 mediates the inhibitory effects of exogenous cholecystokinin octapeptide on cellular morphine dependence[J]. BMC Neurosci,2012,13:63. doi:10.1186/1471-2202-13-63. |
| 17 | HÖKFELT T, MORINO P, VERGE V, et al. CCK in cerebral cortex and at the spinal level[J]. Ann N Y Acad Sci,1994,713:157-163. doi:10.1111/j.1749-6632.1994.tb44062.x. |
| 18 | VÁZQUEZ-LEÓN P, CAMPOS-RODRÍGUEZ C, GONZALEZ-PLIEGO C, et al. Differential effects of cholecystokinin (CCK-8) microinjection into the ventrolateral and dorsolateral periaqueductal gray on anxiety models in Wistar rats[J]. Horm Behav,2018,106:105-111. doi:10.1016/j.yhbeh.2018.10.003. |
| 19 | GOU H, WEN D, MA C, et al. Protective effects of cholecystokinin-8 on methamphetamine-induced behavioral changes and dopaminergic neurodegeneration in mice[J]. Behav Brain Res,2015,283:87-96. doi:10.1016/j.bbr.2015.01.028. |
| 20 | KAUFMANN A, RÖSSLER O G, THIEL G. Expression of the transcription factor Egr-1 in pancreatic acinar cells following stimulation of cholecystokinin or Galphaq-coupled designer receptors[J]. Cell Physiol Biochem,2014,33(5):1411-1425. doi:10.1159/000358707. |
| 21 | LOONAM T M, NOAILLES P A H, YU J, et al. Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum[J]. Life Sci,2003,73(6):727-739. doi:10.1016/s0024-3205(03)00393-x. |
| 22 | WANG H M, DONG J H, LI Q, et al. A stress response pathway in mice upregulates somatostatin level and transcription in pancreatic delta cells through Gs and β-arrestin 1[J]. Diabetologia,2014,57(9):1899-1910. doi:10.1007/s00125-014-3290-0. |
| 23 | YIN W, LI Z, JIN M, et al. A complex structure of arrestin-2 bound to a G protein-coupled receptor[J]. Cell Res,2019,29(12):971-983. doi:10.1038/s41422-019-0256-2. |
| [1] | 陈建波, 郭影, 陈再勇, 鲍人辉, 孔繁荣. 钩吻中毒死亡法医学鉴定1例[J]. 法医学杂志, 2023, 39(5): 509-511. |
| [2] | 龙武, 瞿鹏飞, 马琳, 王蕊, 习严梅, 李玉华, 聂胜洁, 段婷, 杜进良, 唐雪, 赵静峰, 雷普平, 王跃兵. 一起云南不明原因猝死案件中4种野生菌的细胞毒性[J]. 法医学杂志, 2023, 39(2): 121-128. |
| [3] | 张婷婷, 黄钰, 张学军, 陈捷, 花镇东. 污水中甲基苯丙胺干扰物N-甲基-2-苯基丙胺的结构解析[J]. 法医学杂志, 2022, 38(6): 726-732. |
| [4] | 赵龙瑞, 张建波, 韩卫, 朱莉, 陈腾, 官方霖. 整合组学在甲基苯丙胺所致精神障碍法医学鉴定中的应用前景[J]. 法医学杂志, 2022, 38(5): 650-656. |
| [5] | 庄慧娜, 熊进成, 李剑波, 张鹏. 应用虚拟解剖技术分析交通事故死亡原因1例[J]. 法医学杂志, 2022, 38(5): 671-672. |
| [6] | 文岩, 韩昱哲, 龚铎, 解文凯, 吕晨曦, 孟宇珍, 张潮, 尉志文, 贠克明. 呋喃酚葡萄糖醛酸结合物在呋喃丹染毒家兔体内的死后分布及死后再分布[J]. 法医学杂志, 2022, 38(5): 601-605. |
| [7] | 董塔娜, 张磊磊, 刘杰. 服用苯巴比妥东莨菪碱自杀1例[J]. 法医学杂志, 2022, 38(5): 664-666. |
| [8] | 董丽儒, 连俊波, 霍双杰, 罗丹, 杨虎, 宋旭东, 张晓静, 丛斌. 慢性束缚应激对大鼠杏仁核细胞凋亡的影响[J]. 法医学杂志, 2022, 38(4): 459-467. |
| [9] | 向平, 刘宁国, 沈保华, 强火生, 沈敏. 单纯窒息性气体急性中毒死亡案件的检材提取和分析策略[J]. 法医学杂志, 2022, 38(4): 507-514. |
| [10] | 杨柳, 向平, 邓虹霄, 强火生, 党永辉, 施妍, 沈保华. 口服地芬尼多片中毒死亡9例[J]. 法医学杂志, 2022, 38(4): 495-499. |
| [11] | 陈磊昊, 张标, 沈刚. 服用盐酸地芬尼多片中毒死亡4例[J]. 法医学杂志, 2022, 38(3): 420-422. |
| [12] | 严秀莺, 向平, 于治国, 严慧. 代谢组学在滥用物质毒理学研究中的应用[J]. 法医学杂志, 2022, 38(3): 400-407. |
| [13] | 邹亚晶, 姚建, 阚卫军. 溴敌隆投毒致蛛网膜下腔出血伪装猝死1例[J]. 法医学杂志, 2022, 38(2): 293-295. |
| [14] | 严慧, 杜猛, 乔正, 向平, 沈保华, 沈敏, 刘伟. 29例磷化氢中毒者体内总磷化氢分布以及磷化氢中毒特征分析[J]. 法医学杂志, 2022, 38(2): 254-257. |
| [15] | 余延和, 向平, 严慧. 服用石硫合剂自杀1例[J]. 法医学杂志, 2022, 38(2): 291-292. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||